




THE REVOLUTIONARY BIOPTRON PRO 1
Bioptron Pro1 with Table Stand, Bioptron light therapy for home or clinic use
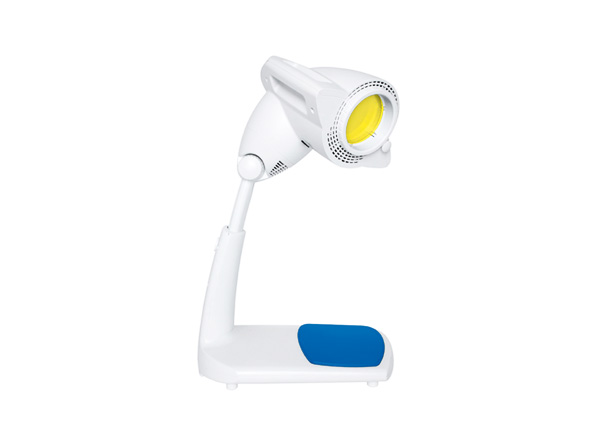
BIOPTRON PRO1 with table stand is a Swiss-made, Class IIa light therapy device designed for people who want consistent, hands-free Bioptron light therapy sessions at home, in clinics, or in rehabilitation settings. With a medium 11 cm treatment filter and an ergonomic table stand, it is built for regular routines across larger areas than smaller handheld models.
Common searches for this device include Bioptron Pro 1, Bioptron light therapy NZ, Bioptron light for sale NZ, Bioptron Pro 1 price, and Bioptron Pro 1 manual. This page is written to help you choose confidently and set up your routine correctly.
What it can be used for (medical device indications)
Bioptron Hyperlight Therapy is certified as a medical device with indications that include:
- Wound healing: venous leg ulcers, pressure sores, and burns
- Pain treatment: rheumatology, physiotherapy, and sports medicine contexts
- Dermatological disorders and skin problems: including acne, herpes, and psoriasis
- Pediatrics and family use: dermal affections in newborns, pediatric musculoskeletal and allergic respiratory disorders, and dermal affections
- Seasonal affective disorders: mild depressions, sleep disorders, and chronic fatigue syndrome
Always read the instructions for use and use as directed. This content is general information, not medical advice. If symptoms persist or you are unsure if Bioptron suits your situation, please consult a health professional.
How Bioptron Hyperlight works
Across the Bioptron device family, the light characteristics are designed to be repeatable and safe for regular use. The PRO1 uses polychromatic light that does not contain UV, and the device is designed to deliver a consistent session when used at the recommended distance.
If you want a deeper explainer, we recommend reading Bioptron Hyperlight and Bioptron Hyperlight Therapy.
How to use it (simple, repeatable routine)
- Session length: typically a few minutes per area, once or twice daily (follow your included instructions for use)
- Distance: the PRO1 is designed for use at a consistent distance (a measuring tool is included to help maintain the recommended 10 cm)
- Hands-free positioning: the table stand helps you keep the angle and distance steady during the session
- Timer control: treatment time can be set on the digital control panel in 30-second intervals
For practical setup, safety checks, and troubleshooting tips, see our Bioptron Support NZ guide.
Why choose the PRO1 with table stand
- 11 cm treatment filter: ideal when you want to cover more surface area than compact models
- Comfortable hands-free use: table stand supports daily routines without holding the device
- Flexible positioning: adjustable height and head inclination, with head rotation for easier targeting
- Swiss-made quality: manufactured in Switzerland, built for repeatable daily use
What is included
Your set includes the BIOPTRON PRO1 device with basic filter, table stand, detachable power cord, protective band for babies’ eyes, protective cover, protective bag, Oxy Sterile Spray, user manual, and warranty.
Choosing between models and setups
If you are comparing options, we recommend starting with the Bioptron Pro1 collection, then comparing treatment area size and stand style. For a compact option, view Bioptron MedAll, and for a larger treatment head, consider Bioptron 2 with Floor Stand.
Benefits of BIOPTRON PRO1 with table stand
Comfort, recovery, and pain relief support
- Designed for repeatable light therapy sessions that can support comfort and recovery routines
- Commonly used in physiotherapy and sports medicine contexts where consistent positioning matters
- Hands-free stand makes it easier to stay relaxed and consistent during sessions
Skin and wound care applications
- Certified medical device indications include support for wound healing (including venous leg ulcers, pressure sores, and burns)
- Certified medical device indications include dermatological disorders and skin problems (including acne, herpes, and psoriasis)
- Non-UV polychromatic light, designed for safe, regular use when used as directed
Built for consistent home use
- 11 cm filter diameter helps you treat larger areas efficiently
- Timer control with 30-second intervals supports repeatable dosing
- Measuring tool helps you maintain the recommended distance, which is key for consistency
Confidence in quality
- Swiss-made device with safety features including protection against overheating
- Backed by a 5-year warranty (with 2 years on bulb and fan, and 5 years on other parts, excluding material damage)
Always read the instructions for use and use as directed. Results vary and depend on many factors including consistency, positioning, and your individual situation.
BIOPTRON PRO1 with table stand FAQs
Does BIOPTRON actually work?
Bioptron is a certified light therapy medical device, and many people use it as part of structured routines for skin, recovery, and pain relief support. Your outcome depends on the condition, consistency, and correct technique.
We recommend starting with clear expectations and a repeatable plan: correct distance, consistent timing, and daily use as directed. For a practical NZ overview, read Bioptron light therapy: benefits, safety, how to use.
What is BIOPTRON used for?
Bioptron is indicated as a medical device for wound healing (including venous leg ulcers, pressure sores, and burns), pain treatment in rheumatology, physiotherapy and sports medicine contexts, and dermatological disorders and skin problems including acne, herpes, and psoriasis.
It is also used across family contexts, including dermal affections in newborns, pediatric musculoskeletal and allergic respiratory disorders, and seasonal affective disorders (mild depressions, sleep disorders, and chronic fatigue syndrome). For a high-level overview of applications, see What Bioptron Treats.
What are the side effects of BIOPTRON light therapy?
When used as directed, Bioptron uses polychromatic light that does not contain UV, and it is designed for safe, non-invasive sessions.
As with any wellness or medical device routine, we recommend extra care if you have a complex condition or take medicines that increase light sensitivity. If anything feels unusual, stop and seek advice. Our step-by-step safety notes are in Bioptron Support NZ.
How often should I use a BIOPTRON lamp?
Most people use Bioptron once or twice per day, depending on the goal and the area being treated, always following the instructions for use included with the device.
The PRO1 makes consistency easier because you can set treatment time in 30-second intervals and use the included measuring tool to keep the recommended distance steady. If you are still choosing a setup, compare options in our Bioptron Pro1 collection.
How do you use a BIOPTRON PRO1 correctly?
Keep the technique simple: clean the area, position the device at the recommended distance, keep the head angle steady, and use a consistent session time.
- Use the table stand to stay hands-free and relaxed
- Use the measuring tool for consistent spacing
- Use the digital timer to repeat the same session length day to day
For a practical home routine checklist, see Bioptron Light NZ guide.
Can I use BIOPTRON at home?
Yes. The PRO1 is designed for both home and professional settings, and the table stand helps you run stable, repeatable sessions without holding the device.
If you want a bigger hands-free setup, compare Bioptron Pro1 with Floor Stand, or if you prefer a more compact option, view Bioptron MedAll.
Can you use BIOPTRON on the face?
Yes, many customers use Bioptron as part of a facial skin routine, following the recommended distance and timing for safe, repeatable exposure.
If your goal is general skin support and appearance routines, start with the fundamentals on Bioptron Hyperlight Therapy, and always follow the instructions for use included with your device.
What is the difference between the table stand and other setups?
The table stand is ideal for seated, hands-free sessions on common areas like knees, shoulders, elbows, hands, or lower legs, where you want stable positioning without moving around.
If you need more reach, easier positioning for full-body areas, or multi-user clinic flow, a floor stand can be more practical. You can compare configurations in the Bioptron Pro1 collection, and explore add-ons in Bioptron accessories.
Where can I learn more about the technology and get the BIOPTRON PRO1 manual style guidance?
Your set includes a user manual, and we also offer easy-to-follow learning resources to help you use the device confidently.
If you want printed references for home use, see Bioptron booklets. For practical setup, safety, and troubleshooting, use Bioptron Support NZ.
General information only, not medical advice. Always read the instructions for use and use as directed. If symptoms persist, consult your healthcare professional.
Tech Data
Tech Data
| ITEM CODE | PAG-990-SAA |
|---|---|
| PRODUCT NAME | BIOPTRON PRO1 with table stand |
| GROSS WEIGHT [KG] | 6.05 |
| NET WEIGHT [KG] | 4.55 |
| WEIGHT WITH STAND | 6.05kg gross / 4.55kg net |
| DIMENSIONS IN BOX | 40 x 30 x 49 cm |
| PRODUCER | BIOPTRON AG - Sihleggstrasse 23, CH-8832 Wollerau - Switzerland |
| MADE IN | Switzerland |
| COLOR | White |
| VOLTAGE | Power supply 100-240 V~, 50/60 Hz |
| POWER CONSUMPTION | 75 W |
| RATED POWER OF HALOGEN | 50 W |
| POWER CORD | detachable |
| LIGHT ENERGY PER MINUTE | an av. of 2.4 J/cm2 |
| PROTECTION AGAINST OVERHEATING | YES |
| POWER DENSITY | on av. of 40 mW/cm² |
| SAFETY CLASS | IIa IP20 |
| SETTING THERAPY TIME | YES |
| WORKING TEMPERATURE | when used 10°C to + 30°C; for storage 0°C to + 40°C |
| WAVELENGTH | 480 - 3400 nm |
| DEGREE OF POLARIZATION | >95% (590 - 1550 nm) |
| COMPOSITION | BIOPTRON PRO1 device with basic filter * Table stand * Power cord (detechable) * Protective band on babies eyes * Protective cover * Protective bag * Oxy Sterile Spray * User manual * Warranty |
| DISPLAY | >YES- digital |
| WARRANTY | 5 Years (2 Years Bulb and Fan, 5 Years on All Other Parts, Does Not Cover Material Damage) |
| CERTIFICATIONS/DECLARATION | * Declaration of Conformity with the medical Directive 93/42 / EEC issued by the producer. *Confirmation of application a medical device in the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products * CE conformity for electrical equipment. * Certificate for Quality Assurance (EN ISO 13485) * Certificate for the Quality Assurance System (Directive 93/42 / EEC) issued by the FDA * DEKRA Certificate for quality control EN ISO 13485:2012 + AC:2012 * DEKRA Certificate for medical devices complying with Annex II, Section 3 of Directive 93/42 / EEC - (Notified Body ID 0124) * Declaration of conformity from DEKRA (European notified body) for all products issued in 2013 (07.21.2013) |
- Choosing a selection results in a full page refresh.



