
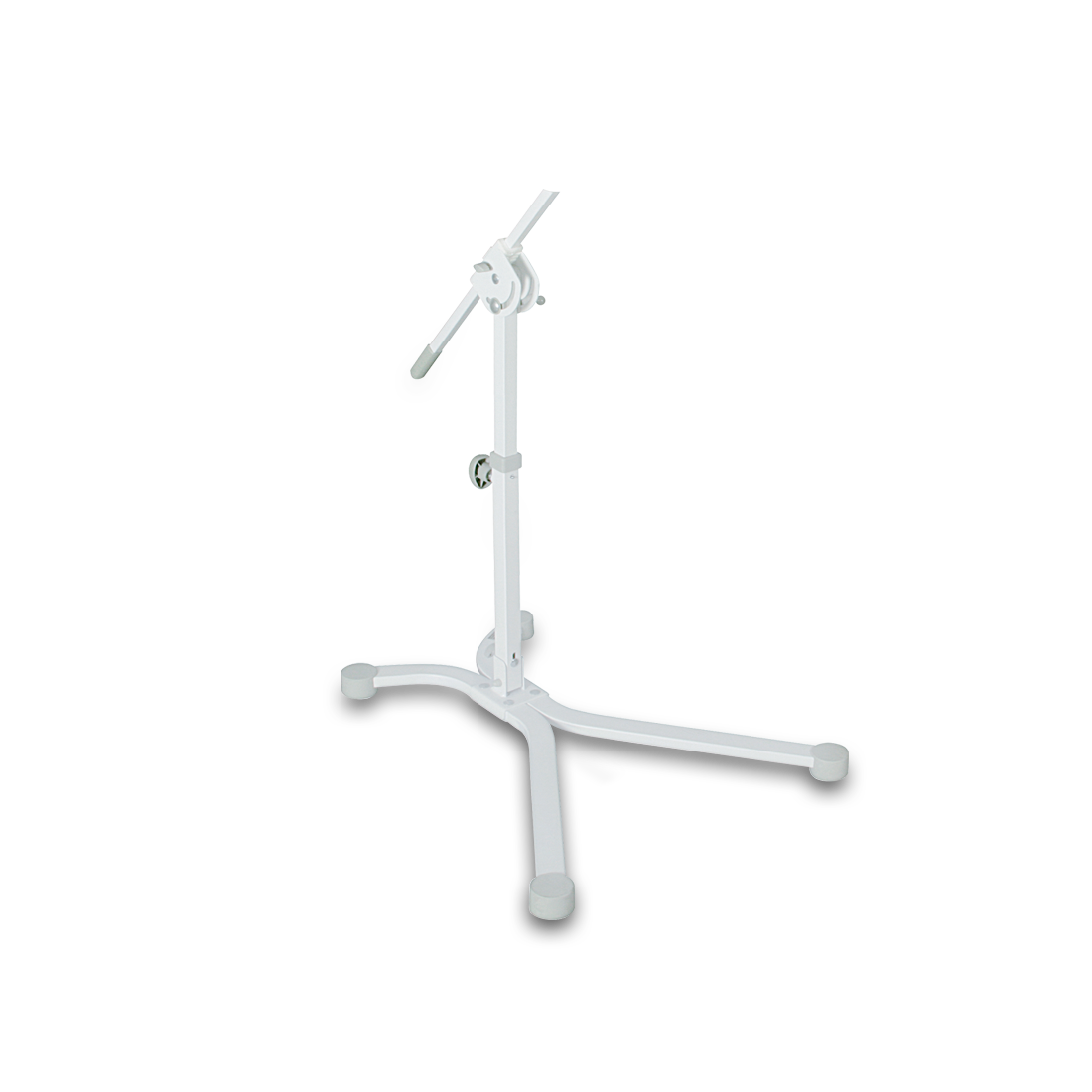



Bioptron Pro 1 with Floor Stand, professional-grade Bioptron light therapy for home or clinic
Bioptron Pro 1 with Floor Stand is a Swiss-made, Class IIa polarized light therapy device designed for people who want consistent, hands-free sessions at home, in clinics, or rehabilitation settings. With an 11 cm treatment filter and an adjustable floor stand, it is built for comfortable routines across larger areas without needing to hold the device.
If you are comparing options like Bioptron Pro 1 price or looking for Bioptron Pro 1 manual guidance, this page is designed to help you choose confidently and use the device correctly from day one.
Why choose the floor stand setup?
- Hands-free positioning: the stand helps you keep the correct angle and distance steady during each session.
- Flexible reach: easily adjust height and head inclination, with up to 360° head rotation for hard-to-reach areas.
- Built-in timer control: set treatment time on the digital control panel in 30-second intervals.
- Distance made simple: a measuring tool is included to help maintain the recommended 10 cm distance.
What it can be used for (medical device indications)
Bioptron Hyperlight Therapy is certified as a medical device with indications that include:
- Wound healing: venous leg ulcers, pressure sores, and burns.
- Pain treatment: rheumatology, physiotherapy, and sports medicine contexts.
- Dermatological disorders and skin problems: including acne, herpes, and psoriasis.
- Pediatrics and family use: dermal affections in newborns, pediatric musculoskeletal and allergic respiratory disorders, and dermal affections.
- Seasonal affective disorders (SAD): mild depressions, sleep disorders, and chronic fatigue syndrome.
For a practical overview of applications, see What Bioptron Treats.
How to use Bioptron Pro 1 (simple, repeatable routine)
- Angle: position the light beam at a 90-degree angle to the area being treated.
- Distance: use the measuring tool to keep approximately 10 cm between the device and the treatment area.
- Time: many routines use short sessions per area (commonly 4 to 10 minutes), once or twice daily, following the included instructions for use.
- Eyes: do not direct the light into open eyes, especially during facial application.
Want to go deeper on how the technology works? Read Bioptron Hyperlight Therapy and Light in Medicine.
What’s included in the box
Your set includes the BIOPTRON PRO1 device with basic filter, floor stand, detachable power cord, protective band for babies’ eyes, protective cover, protective bag, Oxy Sterile Spray, user manual, and warranty.
Delivery, guarantee, and support
We ship from Auckland and include help to get you started with confidence. You can review our Shipping Policy and our 30-Day Satisfaction Guarantee. If you want personalised guidance, reach us via Contact.
General information only, not medical advice. Always read the instructions for use and use as directed. If symptoms persist or you are unsure if Bioptron suits your situation, consult a health professional.
Benefits of Bioptron Pro 1 with Floor Stand
Hands-free consistency (the biggest reason people choose the floor stand)
- More comfortable sessions: stand-based positioning helps you stay relaxed while the device stays steady.
- Repeatable routines: maintain the same angle and recommended distance session after session.
- Easy targeting: adjustable height, head inclination, and 360° rotation support better reach across the body.
Certified medical device applications
- Wound healing indications: including venous leg ulcers, pressure sores, and burns (use as directed).
- Pain treatment contexts: commonly used in rheumatology, physiotherapy, and sports medicine routines.
- Skin indications: dermatological disorders and skin problems including acne, herpes, and psoriasis.
Skin appearance and daily wellbeing support
- Supports daily skin routines: many customers use Pro 1 as part of a consistent skin comfort and appearance routine (results vary).
- Non-invasive and non-UV: polychromatic light designed for regular use when used as directed.
Confidence in quality
- Swiss-made device: built for repeatable day-to-day use.
- Safety features: includes protection against overheating.
- Warranty cover: 5 years (2 years bulb and fan, 5 years on all other parts, does not cover material damage).
Bioptron Pro 1 with Floor Stand FAQs
Does BIOPTRON actually work?
Bioptron Hyperlight is a certified medical device, and many people use it as part of structured routines for wound care, pain management, and dermatological needs. Outcomes vary and depend on correct technique and consistency.
If you want to explore the evidence and real-world experiences, we recommend viewing our Studies page and our Testimonials.
What is Bioptron Pro 1 used for?
Bioptron Pro 1 is indicated for wound healing (including venous leg ulcers, pressure sores, and burns), pain treatment in rheumatology, physiotherapy and sports medicine contexts, and dermatological disorders and skin problems including acne, herpes, and psoriasis.
For helpful starting points by use-case, see What Bioptron Treats, plus the dedicated pages for Wound Care, Pain Management, Dermatology, and SAD.
How do you use Bioptron Pro 1 with Floor Stand?
Use the stand to keep the light steady, at a 90-degree angle to the area, and maintain approximately 10 cm distance (the included measuring tool makes this easy). Many routines use short sessions per area (commonly 4 to 10 minutes), once or twice daily, following the included instructions for use.
- Keep the light still during the session (avoid moving the beam around).
- Do not direct the light into open eyes, especially during facial use.
- Use the built-in timer to keep your routine consistent.
For a simple explainer on the light characteristics, see Bioptron Hyperlight Therapy.
How often should I use a BIOPTRON lamp?
Many people use Bioptron once daily as part of a preventive routine, or twice daily (morning and evening) in medical treatment contexts, following the instructions for use.
If you want personalised guidance for your situation, we can help you choose a routine, contact us via Contact.
What are the side effects of BIOPTRON light therapy, is it safe?
When used as directed, Bioptron uses polychromatic light that does not contain UV and is designed for safe, non-invasive sessions. As with any routine, correct distance, timing, and eye safety matter.
If you want a broader background on how light is used in clinical settings, see Light in Medicine.
Can I use BIOPTRON at home?
Yes. Bioptron Pro 1 is designed for both home and professional settings, and the floor stand makes hands-free, repeatable sessions much easier.
If you are comparing models and setups, browse the range in Bioptron Hyperlight Devices and Sets.
Can you use BIOPTRON on the face?
Yes, many customers use Bioptron as part of facial skin routines, following the recommended distance and timing. The key is to avoid directing the light into open eyes.
For skin-focused applications, visit Dermatology. For add-ons and care items, see Bioptron Accessories.
Can I use Bioptron if I’m pregnant or have a pacemaker?
According to the device guidance, Bioptron light therapy does not interfere with pacemakers, and it can be used during pregnancy, with extra caution recommended in the first 3 to 4 months. For pacemaker use, stand-based sessions are recommended to avoid touching the device during treatment.
If you would like help checking your situation, please reach out via Contact.
What is the difference between Pro 1 with Floor Stand and Pro 1 with Table Stand?
The main difference is how you position the device. The floor stand generally offers easier full-body reach and multi-user flow (useful for clinics or shared households), while a table stand setup can be ideal for seated routines and smaller spaces.
You can compare the table stand configuration here: Bioptron Pro1 with Table Stand.
What’s included, and what about warranty and the 30-day guarantee?
Your set includes the Pro 1 device, floor stand, detachable power cord, protective band for babies’ eyes, protective cover, protective bag, Oxy Sterile Spray, user manual, and warranty. The warranty is 5 years (2 years bulb and fan, 5 years on all other parts, excluding material damage).
For purchase confidence, review our 30-Day Satisfaction Guarantee and Shipping Policy.
General information only, not medical advice. Always read the instructions for use and use as directed. Results vary. If symptoms persist or you are unsure if Bioptron suits your situation, consult a health professional.
Tech Data
Tech Data
| ITEM CODE | PAG-991-SAA |
|---|---|
| PRODUCT NAME | Bioptron Pro 1 with Floor Stand |
| GROSS WEIGHT [KG] | 10.45 |
| NET WEIGHT [KG] | 9 |
| APPLICATION | Professional medical device for the treatment with polarized light |
| PRODUCER | BIOPTRON AG - Sihleggstrasse 23, CH-8832 Wollerau - Switzerland |
| MADE IN | Switzerland/p> |
| FILTER/GLASS DIAMETER | ap. 11 cm |
| COLOR | white |
| VOLTAGE | Power supply 100-240 V~, 50/60 Hz |
| POWER CONSUMPTION | 75 W |
| RATED POWER OF HALOGEN | 50 W |
| POWER CORD | detachable |
| LIGHT ENERGY PER MINUTE | an av. of 2.4 J/cm2 |
| PROTECTION AGAINST OVERHEATING | YES |
| POWER DENSITY | on av. of 40 mW/cm² |
| SAFETY CLASS | IIa IP20 |
| SETTING THERAPY TIME | YES |
| WORKING TEMPERATURE | when used 10°C to + 30°C; for storage 0°C to + 40°C |
| WAVELENGTH | 480 - 3400 nm |
| DEGREE OF POLARIZATION | >95% (590 - 1550 nm) |
| COMPOSITION | BIOPTRON PRO1 device with basic filter * Floor stand * Power cord (detachable) * Protective band on babies eyes * Protective cover * Protective bag * Oxy Sterile Spray * User manual * Warranty |
| DISPLAY | YES - digital |
| WARRANTY | 5 Years (2 Years Bulb and Fan, 5 Years on All Other Parts, Does Not Cover Material Damage) |
| CERTIFICATIONS/DECLARATION | * Declaration of Conformity with the medical Directive 93/42 / EEC issued by the producer. *Confirmation of application a medical device in the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products * CE conformity for electrical equipment. * Certificate for Quality Assurance (EN ISO 13485) * Certificate for the Quality Assurance System (Directive 93/42 / EEC) issued by the FDA * DEKRA Certificate for quality control EN ISO 13485:2012 + AC:2012 * DEKRA Certificate for medical devices complying with Annex II, Section 3 of Directive 93/42 / EEC - (Notified Body ID 0124) * Declaration of conformity from DEKRA (European notified body) for all products issued in 2013 (07.21.2013) |
- Choosing a selection results in a full page refresh.



