




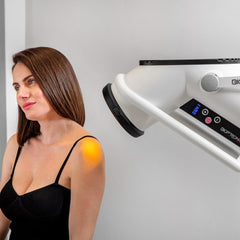
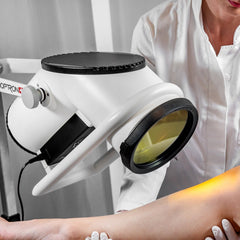



THE PROFESSIONAL CHOICE: BIOPTRON 2 MEDICAL LIGHT THERAPY
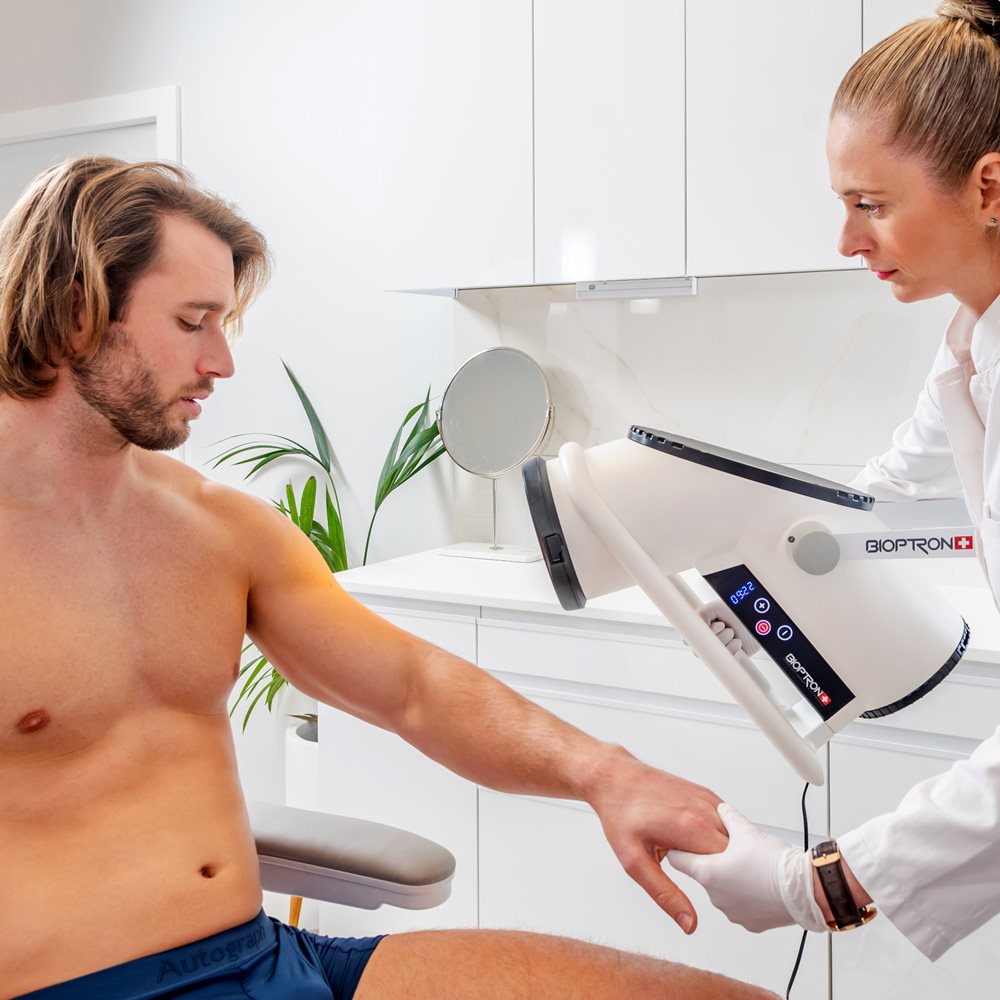
BIOPTRON 2 with Floor Stand, professional Bioptron light therapy for clinics
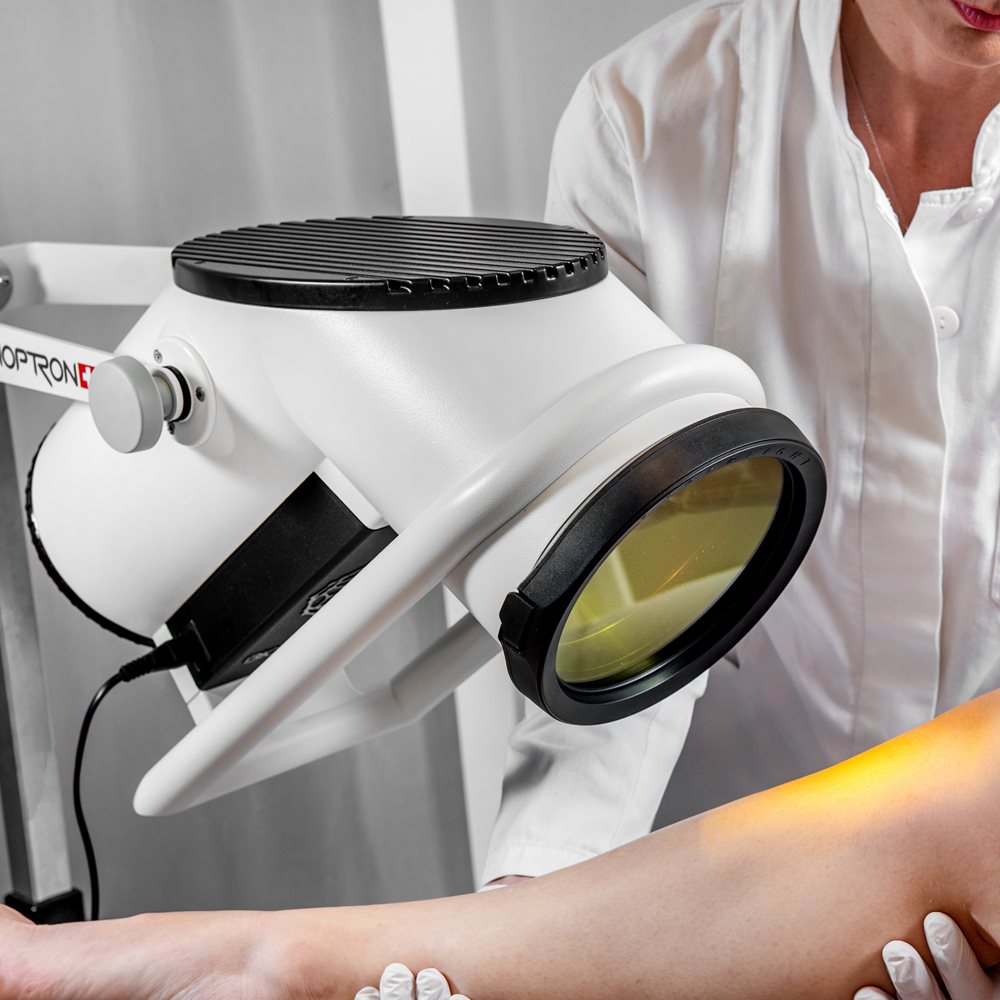
BIOPTRON 2 with floor stand is our larger-format, Swiss-made BIOPTRON Hyperlight medical device designed for hands-free, professional use in clinics, physiotherapy, sports medicine, dermatology, and wound care settings. With a 15 cm filter diameter and an easy-to-position floor stand, it helps you treat larger surface areas efficiently while keeping sessions consistent and comfortable for the patient.
Availability: available for pre-order, delivery upon payment from Auckland.
What it is used for (clinically tested indications)
Our BIOPTRON Hyperlight indication portfolio includes:
- Wound healing: venous leg ulcers, pressure sores, burns
- Pain treatment used in rheumatology, physiotherapy, and sports medicine
- Dermatological disorders and skin problems: including acne, herpes, psoriasis
- Pediatric use: for children and newborns (dermal affections and selected pediatric indications)
- Seasonal Affective Disorder (SAD): light-based support for mild depressions, sleep disorders, and chronic fatigue syndrome
We also see customers choose BIOPTRON as part of broader wellbeing routines (for example, skin vitality and recovery support). Where a use is not a certified medical indication, we describe BIOPTRON as a wellness-support tool.
How BIOPTRON Hyperlight works
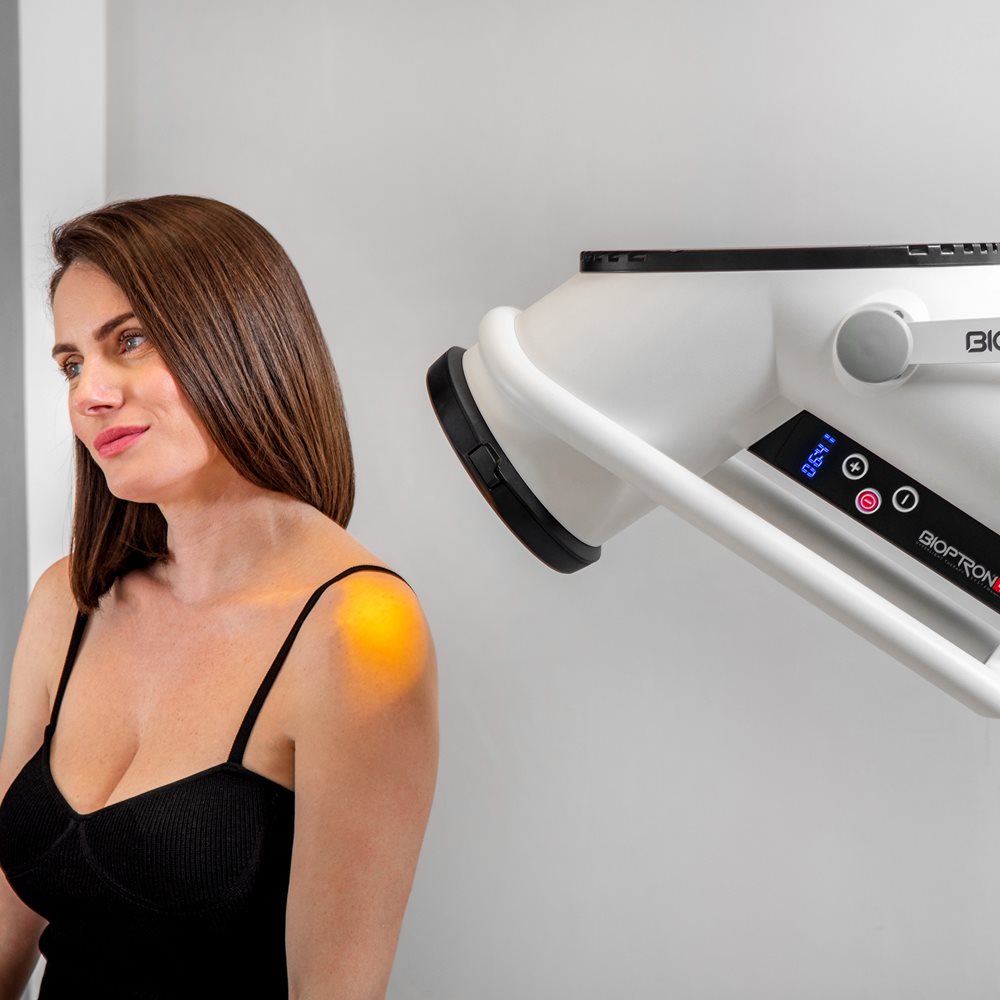
BIOPTRON 2 delivers polarized, polychromatic (multi-coloured), non-coherent light and is designed to be UV-free. In practice, that means you get a controlled, repeatable light dose for targeted areas, without the UV exposure associated with sunlight.
How to use (simple clinical routine)
- Position: hold the light at a 90-degree angle to the area being treated
- Distance: approximately 10 cm from the skin
- Time: commonly 4 to 10 minutes per treatment area (depending on the indication and area)
- Frequency: often once to twice daily, following your protocol or practitioner guidance
The floor stand supports steady positioning (and reduces user fatigue), which is especially useful for repeated treatments and larger zones.
What’s included
- BIOPTRON 2 device
- Floor stand
- Detachable power cord
- User manual
- Warranty
Important note
BIOPTRON sessions and outcomes can vary by person and condition. This content is general information and is not medical advice. For personalised guidance (especially for persistent symptoms, wound care, or mood concerns), please consult a qualified health professional.
Benefits of BIOPTRON 2 with Floor Stand
Designed for efficient professional sessions
- Larger treatment coverage: 15 cm filter supports time-efficient work on broader areas
- Hands-free consistency: floor stand helps keep angle and distance steady for repeatable dosing
- Flexible positioning: angled device design plus stand helps reach different body areas with less setup friction
Clinically tested indication support
- Wound healing support for venous leg ulcers, pressure sores, and burns (as part of an appropriate care plan)
- Pain treatment support used in rheumatology, physiotherapy, and sports medicine settings
- Skin support for dermatological disorders and skin problems including acne, herpes, and psoriasis
- Pediatric suitability for adults, children, and newborns (appropriate protocols and supervision recommended)
- SAD support as a light-based option for seasonal affective disorder related concerns
Comfort, confidence, and everyday practicality
- UV-free light: designed to avoid UV exposure during sessions
- Simple routine: short sessions that fit into clinic workflows
- Long-term value: built for regular use, with a clear, professional-grade setup
Frequently asked questions
What is BIOPTRON light therapy used for?
BIOPTRON Hyperlight is clinically tested and certified for medical treatment indications including wound healing, pain treatment in rheumatology, physiotherapy and sports medicine, dermatological disorders (including acne, herpes, psoriasis), pediatric indications, and SAD support.
If you want a quick overview by category, we recommend our What Bioptron Treats page, and the dedicated sections for Wound Care, Pain Management, and Dermatology.
How do you use a BIOPTRON 2 lamp correctly?
A standard approach is: clean the area, keep the beam at a 90-degree angle, hold the device about 10 cm away, and treat the area without moving the beam.
Most protocols use short, repeatable sessions. For practical education on how the light works, see How BIOPTRON Hyperlight works.
How long should a BIOPTRON session last?
For many indications, a typical session is 4 to 10 minutes per treatment area, depending on the condition and the size of the area being treated.
For SAD-related routines, longer sessions at greater distances may be used. For more context, visit SAD light therapy support.
How often should you use BIOPTRON?
Many people use BIOPTRON once daily for general support or twice daily (morning and evening) when following a treatment routine. Frequency can vary by condition and care plan.
For condition-specific guidance and supporting information, we also share research links on our Studies page.
What are the side effects of BIOPTRON light therapy?
BIOPTRON Hyperlight is designed to be UV-free, and there are no known side effects listed in our standard guidance when used as directed.
During facial use, do not direct the beam into open eyes. If you have a diagnosed eye condition or you are unsure, talk with a health professional first. You can also reach us via Contact for product-use questions.
Can I use BIOPTRON at home, or is BIOPTRON 2 only for professionals?
Many BIOPTRON devices are used both in clinics and at home. BIOPTRON 2 is most commonly chosen for professional settings because it covers larger areas and supports hands-free positioning with a stand.
To compare the range, browse our Bioptron Hyperlight devices collection.
Does BIOPTRON actually work?
BIOPTRON has a clinically tested and certified indication portfolio, and it is used in professional settings worldwide. Results and timelines vary by person, condition, and consistency.
If you want to explore experiences and important disclaimers around personal stories, you can read Testimonials alongside our Studies page.
Does BIOPTRON help with hair loss or skin aging?
Hair loss and skin aging are not listed as certified medical indications in our official indication summary. If you choose to use BIOPTRON for these goals, we recommend thinking of it as a wellness-support tool that may support comfort, skin vitality, and recovery routines rather than a treatment or cure.
For clinically certified skin indications, start with Dermatology.
Is BIOPTRON safe in pregnancy or with a pacemaker?
Our general guidance indicates BIOPTRON can be used in pregnancy, with a recommendation to avoid exposure in the first 3 to 4 months. BIOPTRON does not interfere with pacemakers in standard guidance.
For personalised advice, especially during pregnancy or for implanted devices, please consult your clinician. If you need help choosing a setup or accessories, see Bioptron Accessories and reach out via Contact.
What’s the difference between BIOPTRON 2 and other BIOPTRON devices?
The key differences are coverage size and how you prefer to position the device. BIOPTRON 2 is typically chosen for larger treatment areas and stand-based, hands-free sessions in professional environments.
To compare available options in New Zealand, use our Bioptron Hyperlight devices collection, and if you are ordering from afar, review our Shipping Policy.
Tech Data
| ITEM CODE | PAG-880-SET |
|---|---|
| PRODUCT NAME | BIOPTRON 2 with floor stand |
| GROSS WEIGHT [KG] | 15.56 |
| NET WEIGHT [KG] | 13.27 |
| STAND WEIGHT | 8.80 kg gross/8.20 kg net |
| APPLICATION | Professional medical device for the treatment with polarized light |
| DIMENSIONS IN BOX | Dimensions of the device in package 54,5 x 40 x 25 cm |
| DIMENSIONS IN BOX - STAND | 85 x 94 x 26,5 cm |
| FILTER/GLASS DIAMETER | ap. 15 cm |
| PRODUCER | BIOPTRON AG - Sihleggstrasse 23, CH-8832 Wollerau - Switzerland |
| MADE IN | Switzerland |
| COLOR | White |
| VOLTAGE | Power supply 100-240 V~, 50/60 Hz |
| POWER CONSUMPTION | 1.4 -1.0 A |
| POWER DENSITY | on av. of 40 mW/cm² |
| LIGHT ENERGY PER MINUTE | an av. of 2.4 J/cm² |
| RATED POWER OF HALOGEN | 90 W/p> |
| POWER CORD | Detachable |
| FUSE | T2A/250 V |
| PROTECTION AGAINST OVERHEATING | YES |
| SAFETY CLASS | I |
| WORKING TEMPERATURE | when using 10°C to + 30°C; for storing 0°C to + 40°C |
| WAVELENGTH | 480 - 3400 nm |
| DEGREE OF POLARIZATION | >95% (590 - 1550 nm) |
| COMPOSITION | BIOPTRON 2 device * Floor stand * Power cord (detechable) * User manual * Warranty |
| DISPLAY | YES - digital |
| WARRANTY | 5 Years (2 Years Bulb and Fan, 5 Years on All Other Parts, Does Not Cover Material Damage) |
| CERTIFICATIONS/DECLARATION | * Declaration of Conformity with the medical Directive 93/42 / EEC issued by the producer. *Confirmation of application a medical device in the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products * CE conformity for electrical equipment. * Certificate for Quality Assurance (EN ISO 13485) * Certificate for the Quality Assurance System (Directive 93/42 / EEC) issued by the FDA * DEKRA Certificate for quality control EN ISO 13485:2012 + AC:2012 * DEKRA Certificate for medical devices complying with Annex II, Section 3 of Directive 93/42 / EEC - (Notified Body ID 0124) * Declaration of conformity from DEKRA (European notified body) for all products issued in 2013 (07.21.2013) |
- Choosing a selection results in a full page refresh.



